Featured
- Get link
- X
- Other Apps
Calculating Molality Of A Solution
Calculating Molality Of A Solution. This term is useful in understanding the concentration of a. In this situation, the molarity of a 4 g.
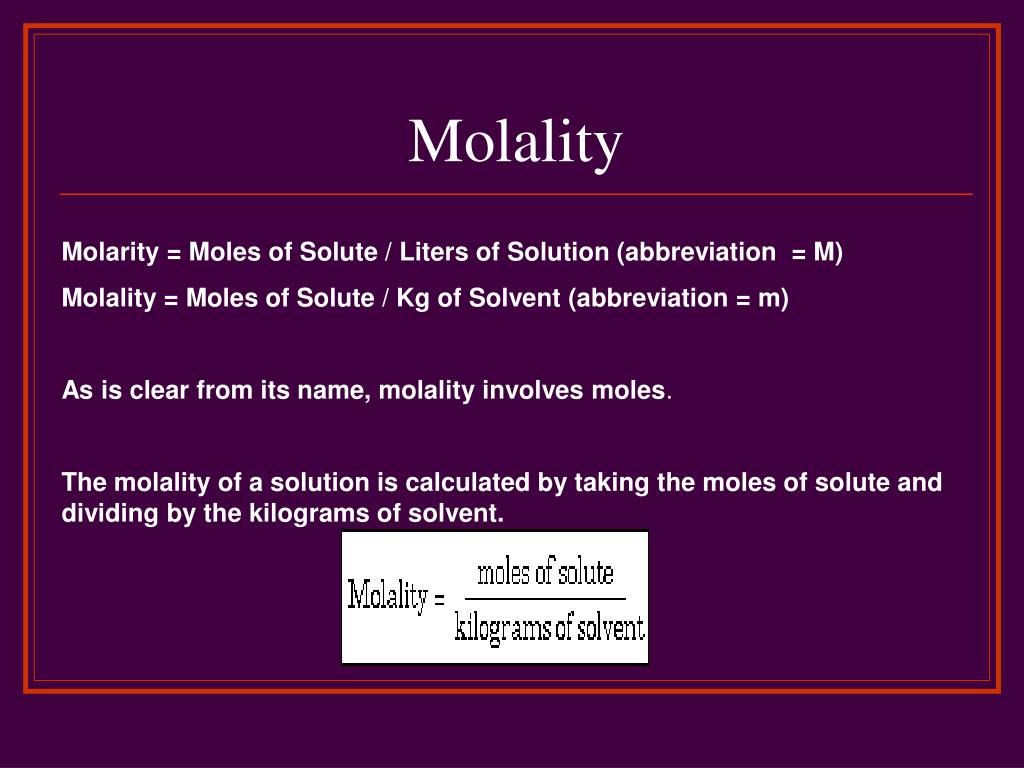
How to calculate molality using this online calculator? In this situation, the molarity of a 4 g. Molality, or molal concentration, refers to the number of moles of solute divided by the mass of solvent, in kilograms, mol∕kg.
The Molality (M) Of A Solution Is Expressed As The Moles (Mol) Of A Solute In A Kilogram (Kg) Of Solvent, The Molality Formula Requires Moles Of Solute And Kilograms Of Solvent.
On the other hand, molality refers to the number of moles of solute. Using molality formula, we have: Step stir until the is completely dissolved.
The Molality (M) Of A Solution Is Defined As The Moles Of Solute Divided By The Kilograms Of Solvent In Which Solute Is Dissolved.
A solution that comprises of 1.0. In other words, molality is the number of moles of solute (dissolved material) per kilogram of solvent (where the solute is dissolved in). In this situation, the molarity of a 4 g.
Molality’s Si Unit Is Moles Per Kilogram Of Solvent.
Molality is an intensive property, and is therefore independent of the amount. Step add water to the until the total volume of the solution is. Molality = moles (solute) ÷ mass (solvent in kg) identify the solute and solvent that make up the solution:
Molality, Or Molal Concentration, Refers To The Number Of Moles Of Solute Divided By The Mass Of Solvent, In Kilograms, Mol∕Kg.
How to calculate molality using this online calculator? The antifreeze in most automobile radiators is a mixture of equal volumes of ethylene glycol and water, with minor amounts of other additives that. It is easy to calculate m if we know the mass of solute and solvent in a solution.
1 Litre Of Solution = 1000 C M 3 = 1000Ml.
Equation used for finding the molarity of a solution is given below; Find the molarity of a sugar solution given the grams of solute (sucrose) dissolved, the volume of solution, and the molecular mass of sucrose (342.2965 grams per mole). Enter the number of moles of solute and weight of solvent, x for the unknown value in the input field.
Popular Posts
How To Calculate How Many Bags Of Concrete
- Get link
- X
- Other Apps
Comments
Post a Comment