Featured
- Get link
- X
- Other Apps
Standard Enthalpy Of Formation Calculator
Standard Enthalpy Of Formation Calculator. Please use the newer page at: The standard enthalpy of formation of substances is here.
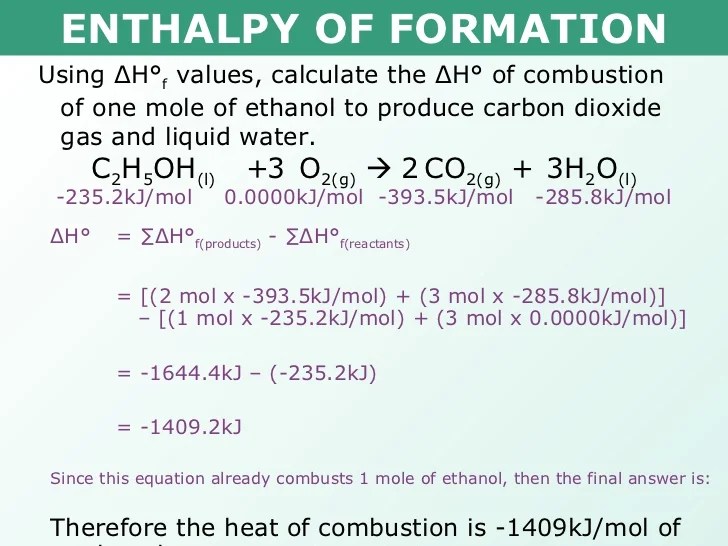
Once ac//°(298.15k) is known, it is possible to derive the standard enthalpy of combustion, ac77°(298.15k), and subsequently calculate the standard enthalpy of formation of the. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of formation is defined as the enthalpy change when 1 mole of compound is formed from its elements under standard conditions.
223 Rows The Standard Enthalpy Of Formation Or Standard Heat Of Formation Of A Compound Is The Change Of Enthalpy During The Formation Of 1 Mole Of The Substance From Its Constituent.
As with the products, use the standard heat of formation values from the table, multiply each by the stoichiometric coefficient, and add them. I think your question isn't totally clear. Δh = 3267 + 6.
From The Standard Enthalpies Of Formation, Calculate $\Delta H_{\Mathrm{Rxn}}^{… 06:56.
Working out an enthalpy change of reaction from enthalpy changes of formation this is the commonest use of simple hesss law cycles that you are likely to come across. Please use the newer page at: This form has bee retired.
Use Equations A And B To Determine $\Delta H$ For The Following Reaction.
Hess's law says that the enthalpy changes on the two routes are the same. Δ f h ° = − 74.81 k j m o l − 1. The standard enthalpy of formation at 25c 29815 k for 1 mol of the substance in its given state g gas and l liquide from its elements in their standard state stable forms at 1.
Calculate The Standard Enthalpy Of Formation Of Carbon Disulfide (Cs2) From Its Elements, Given The Following Data.
However, we can use standard enthalpies of. For most chemistry problems involving δh_f^o, you need the following. Using δh f 0 to calculate δh rxn.
However, An Online Chemical Equation Balancer Calculator Will.
(5 pts) \\[ \\begin{array}{ll} 2 \\mathrm{mg. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. Calculate the standard enthalpy of formation of solid magnesium hydroxide, given the following data:
Popular Posts
How To Calculate How Many Bags Of Concrete
- Get link
- X
- Other Apps
Comments
Post a Comment