Featured
How To Calculate Percent Recovery Chemistry
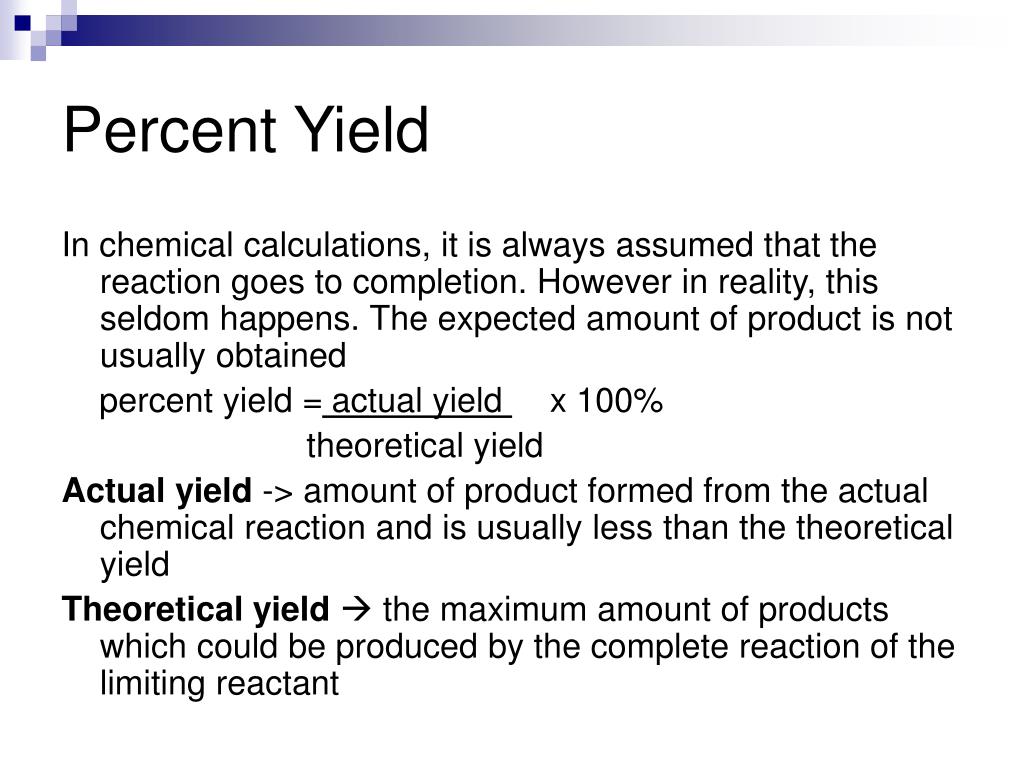
How To Calculate Percent Recovery Chemistry. Percent yield is the amount of a compound obtained from a chemical synthesis reaction with respect to the theoretically. In chemistry, the percent recovery formula is:
Start with the pure, known iron compound (fe) and calculate its number of moles. Percent yield is the amount of a compound obtained from a chemical synthesis reaction with respect to the theoretically. Assume you had 10.0 grams of impure.
From The Equation, Calculate The Number Of Al Moles You Would Have Required.
If you need to calculate it to obtain a measure of your purification, divide the mass of the compound you purified by the mass of the same compound prior the purification and multiply. When purifying a substance in chemistry, use (collected mass/starting mass)*100 to calculate percent recovery. What is a good recovery percentage?
When You Look Up Cheem Research Papers, The Percentage Yield Of Each Reaction Is Written Above The Arrow.
Weight of compound you started with. Start with the pure, known iron compound (fe) and calculate its number of moles. Moreover, i want to know the formula both for spiked concentration and.
In Chemistry The Percent Recovery Formula Ispercent Recovery Weight Of Compound Recovereddivided Byweight Of Compound You Started Withmultiplied By 100.
Search by textbook, topic, or keyword. This formula is also commonly stated as (pure product. Example, in an experiment, 6.527g of zinc chloride yielded 2.935g of zinc metal.
Percent Yield Is The Amount Of A Compound Obtained From A Chemical Synthesis Reaction With Respect To The Theoretically.
Organic chemistry practice problems and problem sets. To compute for percentage recovery, four essential parameters are needed and these parameters are weight recovered of concentrate (c), assay of concentrate (c), weight. Consequently, how do you calculate percent recovery in chemistry?
Membrane Systems Operating At 82% Recovery Will Convert 82% Of The Total Raw Water Input Into Treated Permeate, With The Remaining.
The image above represents percentage recovery (tailings). Quality practice questions and problem sets for organic chemistry. Identify the theoretical yield for the given chemical reaction.
Popular Posts
How To Calculate How Many Bags Of Concrete
- Get link
- X
- Other Apps
Comments
Post a Comment